

Class 11th Biology textbook comprises some of the most important topics for students to prepare for entrance exams. One of the chapters, which is relatively more accessible than the others, is Class 11 Biology Chapter 9 Biomolecules. Our team of subject-matter experts at Vedantu prepared Biomolecules Notes for Class 11 students' convenience. In Class 11 Biology Chapter 9 Notes, you will learn about different types of biomolecules present in the human body and other living organisms. Also, you will find out how various biomolecules play vital roles in our daily life. Students can download a free PDF of CBSE Class 11 Biology Revision Notes for Chapter 9 Biomolecules through the PDF link below.
Also, check CBSE Class 11 Biology revision notes for other chapters:
CBSE Class 11 Biology Chapter-wise Notes
Chapter 9: Biomolecules Notes
Biomolecules Basic Related Other Study Materials
Competitive Exams after 12th ScienceSection–A (1 Mark Questions)
1. How many classes of biomolecules are there?
Ans. There are four major classes of biomolecules – Carbohydrates, Proteins, Nucleic acids and Lipids.
2. Name two basic amino acids.
Ans. Lysine and Arginine are two examples of basic amino acids.
3. Which is the most abundant component found in living organisms?
Ans. Water is most abundant component found in living organisms.
4. Why are enzymes called biocatalysts?
Ans. Enzymes are biocatalysts because they speed up the rate of biological reactions.
5. Draw the structure of the amino acid alanine.
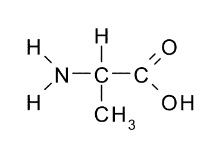
Section–B (2 Mark Questions)
6. The two strands in DNA are not identical but are complementary. Explain.
Ans. In the double helical structure of DNA, Specific pairs of bases in two strands are held by hydrogen bonds (Adenine forms two hydrogen bonds with thymine, while cytosine forms three hydrogen bonds with guanine). One strand runs in 3’-5’ direction while the other runs in 5’-3’ direction. So, the two strands of polynucleotides are antiparallel i.e., run in the opposite direction.
7. Name any two denaturing factors for enzymes?
Ans. Temperature: Extreme high temperatures can cause an enzyme to lose its shape (denature) and stop working.
pH: Changes in pH affect the chemistry of amino acid residues and can lead to denaturation of enzymes.
8. What is the effect of denaturation on the structure of enzymes?
Ans. Most of the enzymes are proteins. In conditions of denaturation there would be no change in the primary structure of the protein. In the secondary and the tertiary structure of proteins, helixes get uncoiled and as a result of this secondary and the tertiary – structured proteins loose theory three-dimensional molecular structure. The loss of secondary and the tertiary structure causes enzymes to lose their catalytic activity.
9. Write two main functions of carbohydrates in plants.
Ans. The two main functions of carbohydrate in a plant are:
(i) Polysaccharides like starch act as a storage molecule.
(ii) Cellulose is used to build the cell wall, and it is a polysaccharide
10. Select the most appropriate chemical bond among ester bond, glycosidic bond, peptide bond and write against each of the following.
Ans. (i) Polysaccharide- glycosidic bond
(ii) Protein- peptide bond
11. Where is the location of phosphodiester bonds in a DNA?
Ans. A phosphate moiety links the 3’-carbon of one sugar of one nucleotide to the 5’-carbon of the sugar of the succeeding nucleotide. The bond between the phosphate and hydroxyl group of sugar is an ester bond. As there is one such ester bond on either side, it is called phosphodiester bond.
The living organisms produce an organic molecule called a biomolecule that helps in performing important functions and acts as a building block of life. They are composed of carbon, nitrogen, hydrogen, phosphorus, oxygen, and Sulphur. There are four types of biomolecules that are common, they are proteins, carbohydrates, nucleic acid, and lipids.
The living tissues are treated with trichloroacetic acid by grinding them to make a slurry that is helpful in analyzing the chemical organic compound and its composition while the tissue should be burned to form ashes in the case of the analysis of the inorganic chemical composition, a sample of tissue should be burnt to obtain ash.
In living organisms, there are essential substances that are important and belong to any class of nitrogenous organic compounds having large molecules that are made up of several long chains of amino acids.
The proteins are composed of amino acids that act as their building blocks. Naturally, around 22 amino acids are found that are made up of hydrogen, carbon, oxygen, and nitrogen. So, one amino acid is composed of the amino group, hydrogen atom, carboxyl group, and distinctive side chain that are bonded with the alpha-carbon.
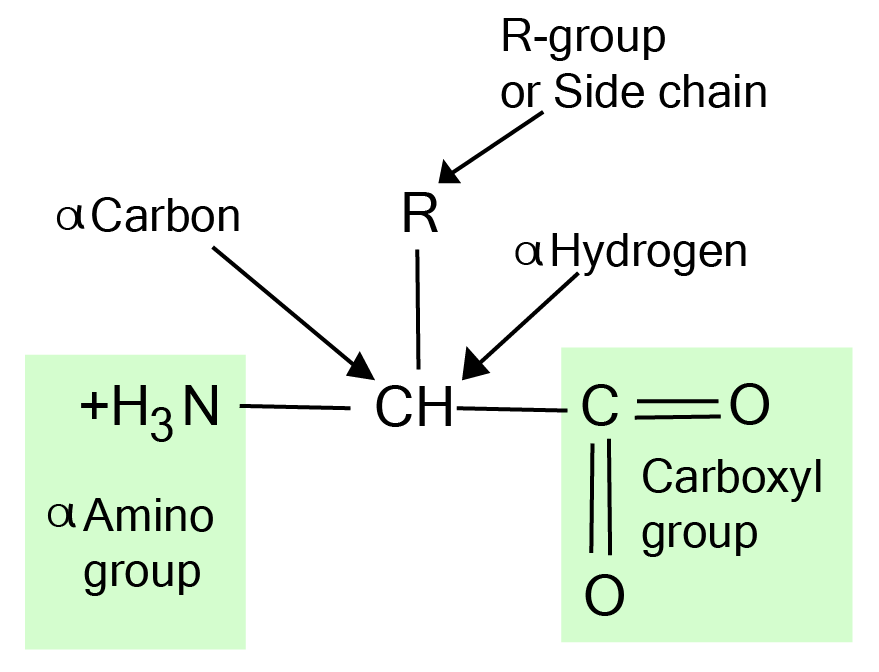
Fig.1. structure of amino acid
The amino acids are present in the form of dipolar ions or zwitterion in solutions when they get dissolved in the water. They function either as proton donors or proton acceptors.
Fig. 2. Zwitterion
All amino acids are found to rotate along the plane based on the polarized light and are said to be optically active. They consist of chiral carbon except for glycine. A chiral carbon is one having four different constituents in a tetrahedral carbon atom.
Two or more amino acids together constitute a peptide and are linked with the help of the peptide bond that is said to form a dipeptide. A tripeptide is formed when three amino acids are joined together while an oligopeptide chain is formed when 12 to 20 amino acids are joined together. When many amino acids are joined together they result in the formation of polypeptides. The first amino acid in the polypeptide chain is known as the N terminal or amino-terminal and the last amino acid in the polypeptide chain is known as the C terminal or carboxyl-terminal.
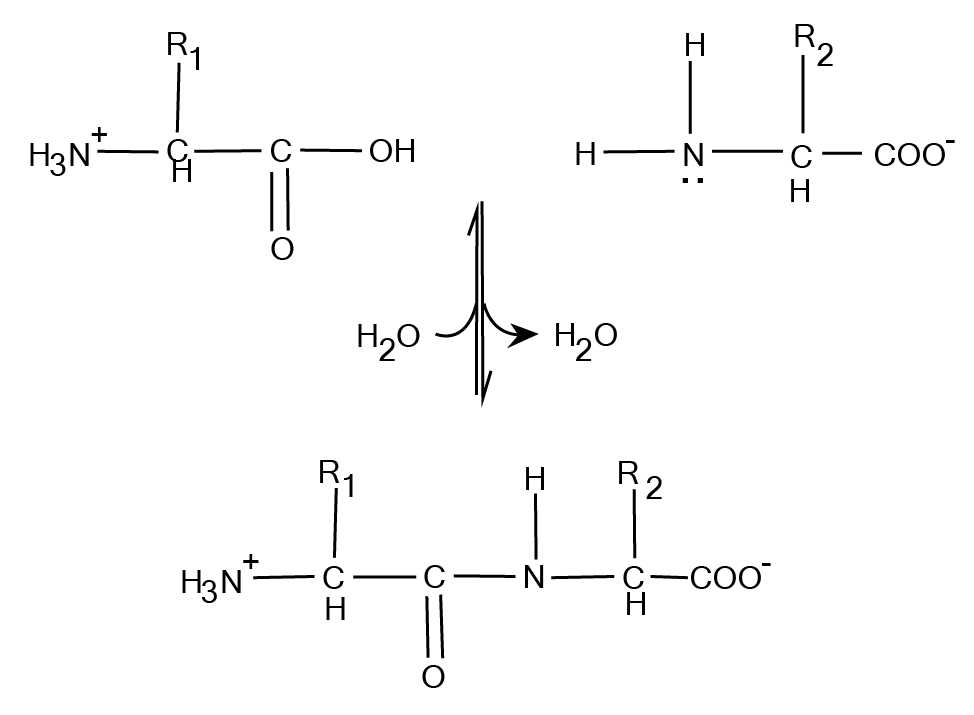
Fig. 3. Formation of peptide bond
There are four levels of protein organization in proteins. They are as follows:
(i) Primary structure: This structure of the protein consists of the amino acids sequence that is joined together by a peptide bond.
(ii) Secondary structure: This stricture is a protein organization having a higher level that is comprised of the alpha-helix and the beta-sheets. These both are stabilized due to the presence of hydrogen bonds in between the carbonyl and N-H groups in a polypeptide backbone.
(iii) Alpha helix: When a polypeptide chain twists, it will form a rigid, rod-like structure that changes into a helical conformation.
(iv) Beta pleated: The arrangement of two or more polypeptide chain segments that are side by side then it results in the formation of a sheet, and every chain segment is known as beta-strand.
(v) Tertiary structures: A three-dimensional structure of protein due to the interaction between the primary structure and its side chains. To make them stabilized, they required hydrophobic interactions, hydrogen bonds, electrostatic interactions, van der Waals forces, and covalent bonds.
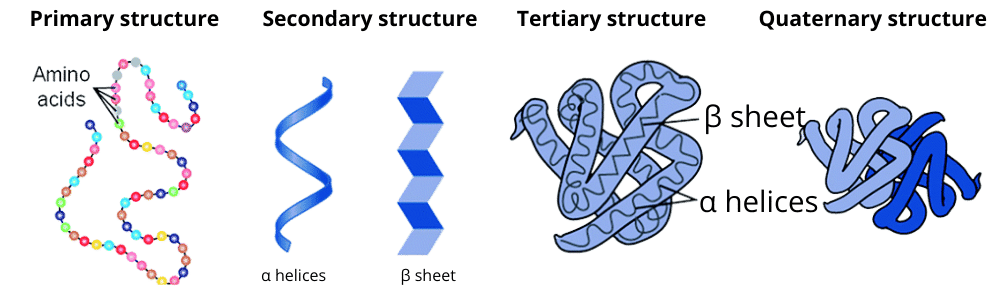
Fig. 4. Levels of protein organization
(vi) Quaternary structures: They are made up of two or more polypeptide chains that are joined together with the help of hydrophobic interactions, hydrogen bonds, electrostatic interactions, etc. For example, hemoglobin.
(vii) Fibrous and globular proteins
Fibrous proteins are long, rod-shaped molecules that are found to be insoluble in water and are structural and protective in nature while the globular proteins are water-soluble and are made up of spherical-shaped molecules.
Friedrich Miescher was the first to discover nucleic acid from the nuclei of pus cells. The nucleic acids are of two types: deoxyribonucleic acids (DNA) and ribonucleic acids (RNA).
In nucleic acid, the nucleosides are the monomeric unit consisting of three constituents: - a nitrogenous base, a five-carbon sugar, and phosphoric acid.
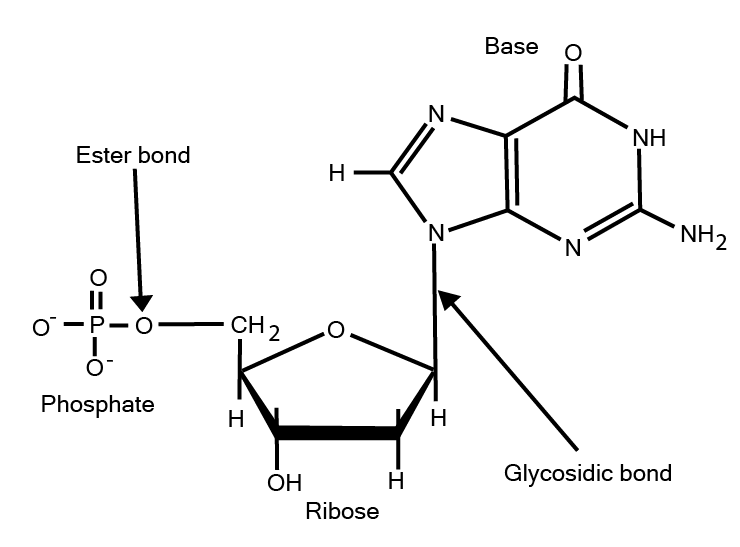
Fig. 5. Structure of nucleotide
The nitrogenous bases are aromatic molecules having a heterocyclic structure. Basically, two types of nucleic acids are found, that are purines and pyrimidines.
Purines are of two types- adenine and guanine while pyrimidines are of three types thymine, cytosine, or uracil.

Fig 6: Structure of purines and pyrimidines
DNA is deoxyribose that constitutes 5- carbon sugar while RNA is ribose. Nucleoside when present with phosphate is known as the nucleotide.
There are two different types of DNA present, such as:
• The two strands of the long polynucleotide are found that are coiled around the axis.
• The two strands are arranged in an antiparallel manner to each other.
• Nucleoside adenine base pairs with thymine while guanine base pairs with cytosine.
• Two hydrogen bonds are formed between adenine and thymine while between guanine and cytosine three hydrogen bonds are formed.
• The structure of this DNA is thinner in comparison to B DNA.
• The purine and pyrimidine bases are arranged alternatingly.
• The DNA constitutes of high salt concentration to make the structure stabilized.
The two strands of DNA separate out when they are exposed to the varying pH, temperature, etc, the condition is called denaturation. To regain the original structure of DNA, the denaturant such as pH, temperature are to be removed, this condition is known as renaturation or annealing.
Fig. 7. Process of denaturation
RNA is called ribonucleic acid having a single-stranded stricture. They are found in various forms inside the cell, the forms are:
(i) Messenger RNA: Their main function is to carry the genetic information from the DNA of the parent to the DNA of the next generation in the form of codons (three nucleotides form a codon), which helps in the coding of the amino acids or protein.
(ii) Transfer RNA: Their main function is to synthesize protein. They are intermediate and helps in the interaction between the language of the nucleic acid and protein, and are located inside the cytoplasm of the living cell.
(iii) Ribosomal RNA: It constitutes ribosomes that play a major role in the synthesis of proteins in the case of both the eukaryotes and the prokaryotes.
The polyhydroxy aldehydes or polyhydroxy ketones come under this category and are made up of carbon, hydrogen, and oxygen. Monosaccharides like glucose are the simplest carbohydrates known. The disaccharides are those carbohydrates that are made up of two monosaccharides joined together while oligosaccharides are formed by the polymerization of 2 to 10 units of monosaccharides. The polysaccharides are formed when hundreds to thousands of monosaccharides are joined together. Monosaccharides that consist of the aldehyde group are known as aldoses while the ketone group is known as ketoses. The simplest monosaccharides are trioses.
The energy in plants is stored in the form of starch that is made up of several units of glucose and is a branched structure. It is made up of two different polymers amylose and amylopectin. The unbranched polymer is amylose in the glucose units while the branched polymer is amylopectin in glucose units.
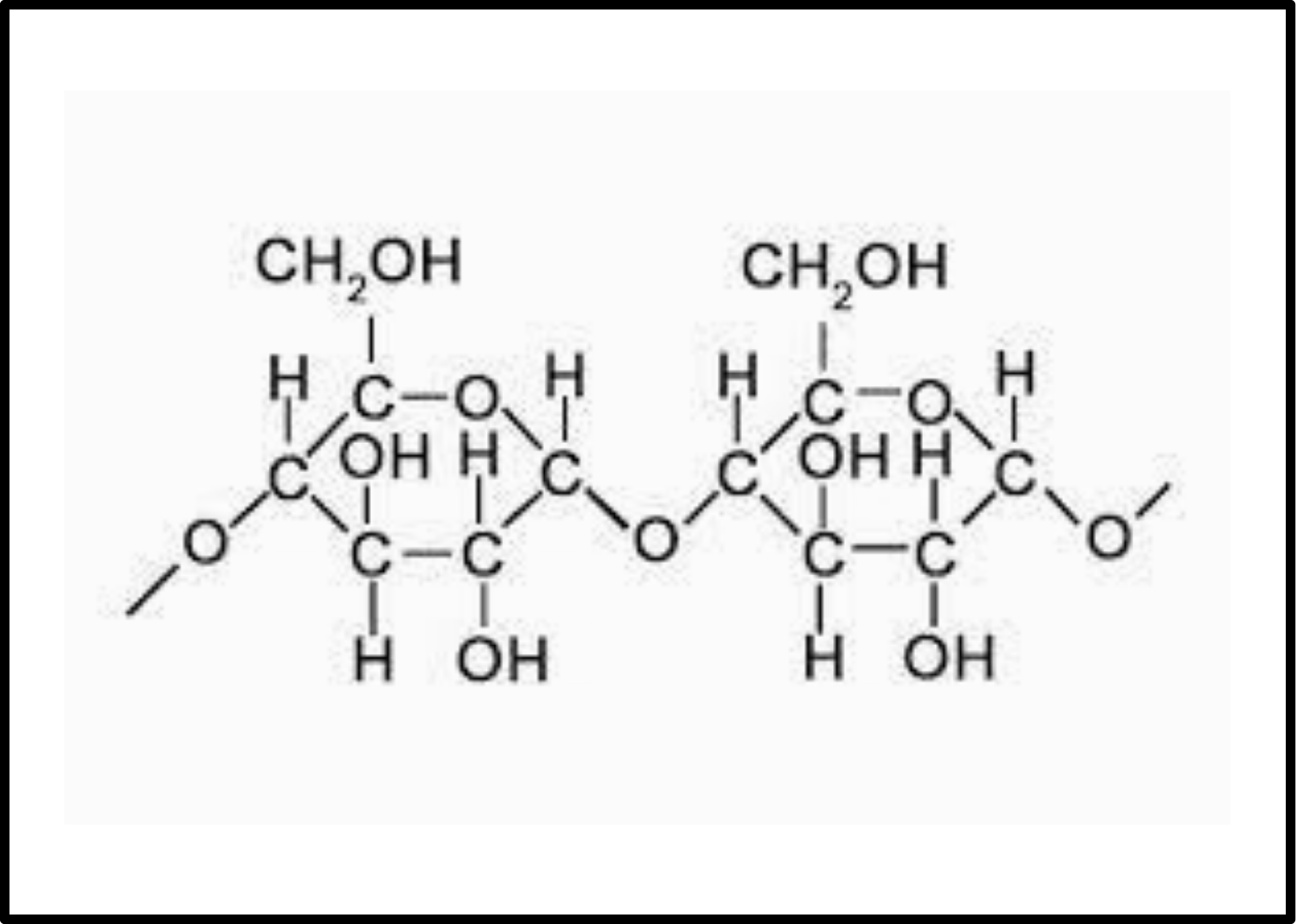
Fig. 8. Structure of starch
In the case of animals, the energy is stored in the form of glycogen in muscle and liver. It is also made up of two different polymers amylose and amylopectin having a highly branched structure of the polymer.
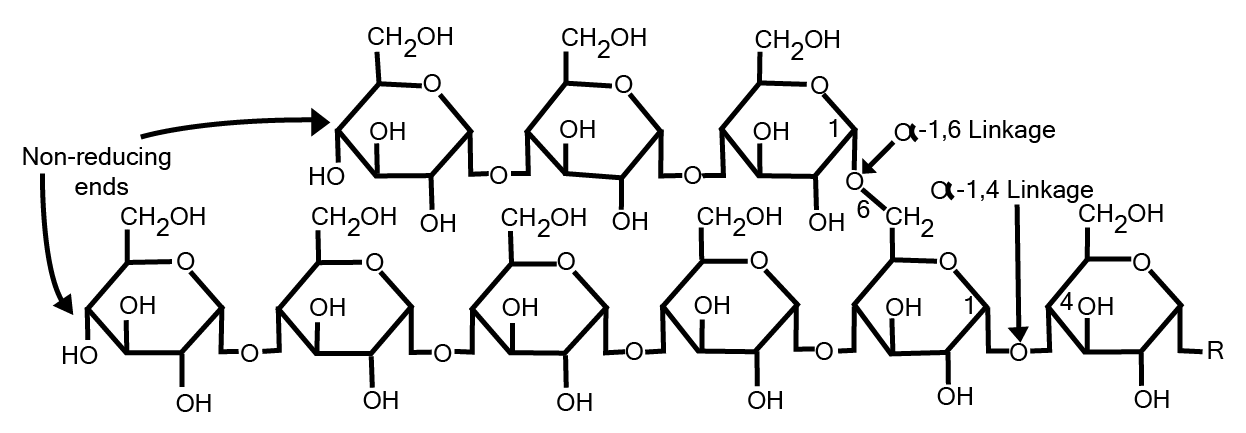
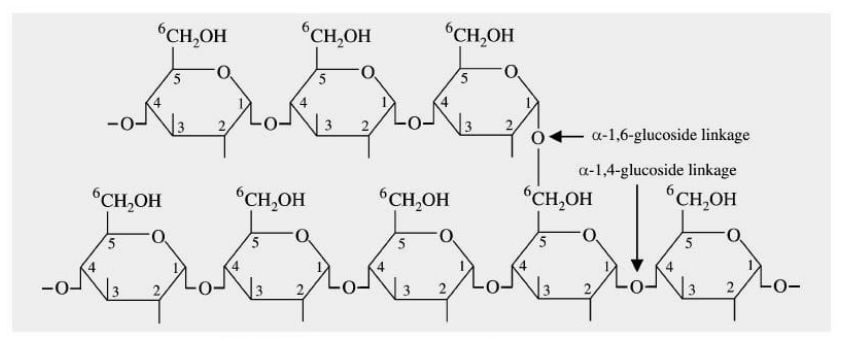
Fig. 9. Structure of glycogen
The unbranched polymer is cellulose in the glucose units which in plant cells, is a structural polysaccharide. In the biosphere, it is the most abundant organic molecule that is present in the plant cells and helps in providing strength and rigidity to the cell.
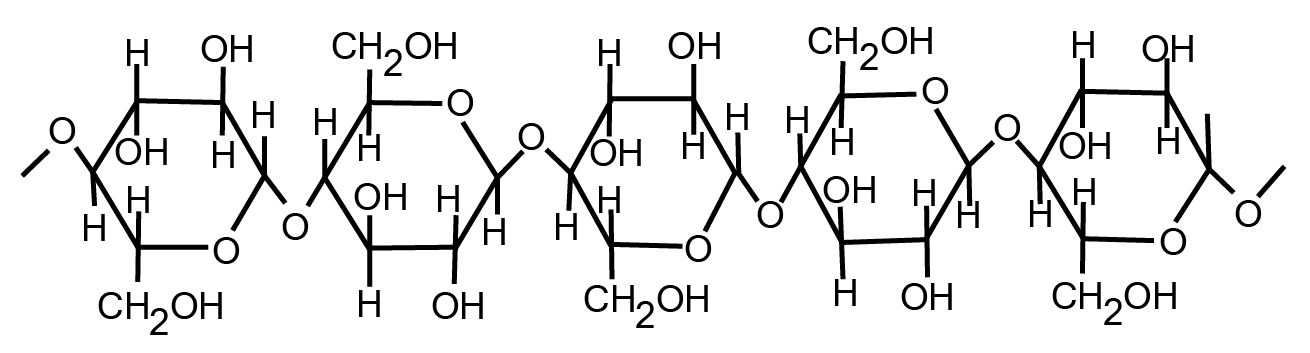
Fig. 10. Structure of cellulose
The exoskeleton of insects and crustaceans is made up of a linear polysaccharide called chitin which is composed of N-acetyl-D-glucosamine residues.
Reducing sugars are those that help in reducing the ferric or cupric ions. Examples include all monosaccharides.
Non-reducing sugars are those that do not help in reducing the ferric or cupric ions. For example, sucrose.
The compounds that are poorly or completely insoluble in water are called lipids but are soluble in other non-polar solvents like chloroform, ether, or benzene.
• Their main function is to store energy which can be utilized later.
• They are the major component in the structure of the membranes.
• Their main function is to protect plants, bacteria, insects, and vertebrates.
The simplest form of lipids are fatty acids and are made up of long chains of hydrocarbons along with one carboxyl group. The alkyl chain present in it may either be saturated or unsaturated. There are one or more than one double bonds resent in the case of the unsaturated fatty acids having both the polar and non-polar ends. Fatty acids such as linoleate are unable to form in the case of mammals. The fatty acids that are called essential fatty acids are not synthesized in the body but are essential so they must be obtained from the diet while some fatty acids that are synthesized in the body are called non-essential fatty acids.

Fig. 11. Structure of fatty acids
Triacylglycerol is also known as triglycerides is non-polar, hydrophobic in nature, and is made up of esters of fatty acids and glycerol.
Enzymes are made up of proteins and consist of various structures like proteins that include the primary, secondary, and tertiary structures. The enzyme consists of an active site that helps in binding the substrate molecule. The properties of the enzymes are as follows:
• All enzymes are proteins, but all proteins are not enzymes.
• For each substrate, the enzymes are specific.
• Enzymes function as catalysts.
• During the reaction, the enzymes are not used up.
• They are of six major types: oxidoreductases, transferases, hydrolases, lyases, ligases, and isomerases.
• For an enzyme to function, some of them require a cofactor and/or a co-enzyme to function.
• Co-factor: They are the non-protein constituents that when binds to an enzyme will make them catalytically active.
• Co-enzyme: They are the organic compounds that during the course of the reaction will binds to the enzyme transiently.
• Prosthetic groups: they are the organic substances that are bounded to the enzyme very tightly.
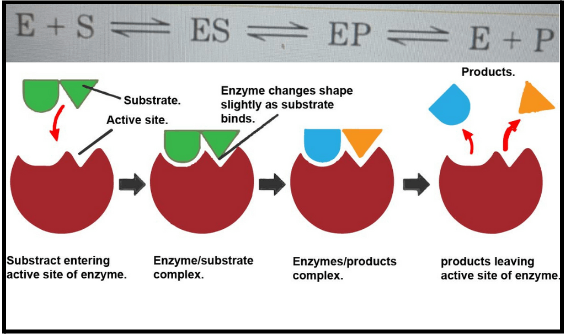
Figure 12: Enzyme-substrate activity
The enzyme activity can be affected due to various factors that include temperature, pH, and substrate concentration. The optimum temperature needs to be maintained or the high or low temperature will lead to the inactivation of the enzyme activity. The enzyme activity can be altered due to deprotonation or protonation.
The molecules such as the inhibitors are responsible for the inactivation of the enzymes. Competitive inhibitors are the most common type of inhibitors that compete to bind to the active site of the enzyme against the substrates. For example, with the help of Malonate, the inhibition of succinate dehydrogenase can be done.
If we look at the biomolecules and see which of them are present inside our bodies, we will be shocked to know that there are not more than four significant types of biomolecules and all the other biomolecules fall under these four categories. The four biomolecules are well explained in the Class 11 Biology Chapter 9 Notes, for your perusal.
Proteins - These are said to be the prime building material present in the human body. From your hair, skin, muscles to macro and micro organs, all are made from proteins. In addition to this, proteins have the property, which makes them strong and yet flexible. However, they have a complex 3-D structure. Proteins are formed when amino acids come together. The amino acids are from the amine group compound on one end and the other end's carboxyl group compound. Thus, it becomes acidic.
Fats - They are also known as lipids, and they help living organisms store energy for long. Fat molecules are relatively compact, and they are lightweight too. As a result, they work as a sponge to absorb and store excess amounts of energy.
Carbohydrates - Glucose is a common word for carbohydrates. A common form of carbohydrates is sugar, a quick energy source as it is easily broken down. The more complex carbohydrates comprise the glucose subunits, which are strung together by di-saccharides, tri-saccharides, and poly-saccharides.
Nucleic Acid - These are the ones that contain genetic codes and have all the information that is required to build a body. The nucleotide is the basic unit of nucleic acid, composed of sugar and phosphate structures.
The features that make living organisms different from non-living organisms are growth, breathing, reproduction, digestion, ability to sense, etc. Despite having non-living parts present inside the body, how can a living organism perform all the tasks that make him fall under the category of a living being? With the help of Class 11, Chapter 9 Biology notes, you will get to know how the living body works. Also, what biomolecules help a living body perform its daily tasks.
In Class 11 Biomolecules notes, you will know about the typical fundamental relationship between structure and function. You will also understand how all biomolecules are influenced by factors such as the environment surrounding them, making the creation of molecules possible.
Moreover, in Class 11 Chapter 9 Biology notes, you will be studying monosaccharides and disaccharides' structures along with the chemical reactions of glucose. Likewise, the Chapter 9 Biomolecules Class 11 notes will give you a step-by-step procedure of naming different biomolecules and classifying them in groups. Lastly, students will learn about the biological function of nucleic acids.
Read the points below and know why it is important for the students to refer to Vedantu’s Class 11 Biology Chapter 9 Revision Notes.
Revision Notes on Class 11 Biology Chapter 9 Biomolecules are the best study material available for students preparing for Class XI Examination. These revision notes are helpful for the students to improve their understanding of concepts. Besides this, you will also find NCERT solutions for Class 11 Biology Chapter 9 Biomolecules on Vedantu's official website in PDF format. This will help you build the knowledge and skills to solve the questions asked in the exam easily and accurately.